Matter is anything that takes up space. It is classified by properties or characteristics including:
STATE
MASS
WEIGHT
VOLUME
DENSITY
MAGNETISM
CONDUCTION
Contributors
Wednesday, March 26, 2008
Thursday, March 13, 2008
Classification of Matter
Matter can be classified into many things:
Element: substance made up of only one kind of atom
Molecule: smallest number (group) of atoms that make up a compound
Compound: substance made of two or more elements
Mixture: two or more substances blended together; because they do not make a new substance, thay keep their own physical properties; can be seperated
Solution: mixtures that are blended so well that their properties are the same throughout; one substance dissolves into the other
Element: substance made up of only one kind of atom
Molecule: smallest number (group) of atoms that make up a compound
Compound: substance made of two or more elements
Mixture: two or more substances blended together; because they do not make a new substance, thay keep their own physical properties; can be seperated
Solution: mixtures that are blended so well that their properties are the same throughout; one substance dissolves into the other
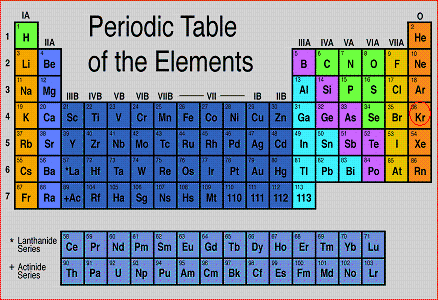
Periodic Table
The periodic table was made to show data of all elements. It contains the element's chemical symbol, atomic number, atomic mass, and the element's name. It is arranged in rows in increasing atomic number. Elements in the same collumn are similar.
States of Matter
State
Solid
Liquid
Gas
Atom Movement
Small Distances
Larger Distances
Greater Distances
Energy
Low
Medium
High
Shape
Fixed
Container's Shape
Fills Space
When matter absorbs energy, its atoms move faster and they spread apart from each other. Atoms are packed closest together in solids and farthest apart in gases.
Solid

Liquid

Solid
Liquid
Gas
Atom Movement
Small Distances
Larger Distances
Greater Distances
Energy
Low
Medium
High
Shape
Fixed
Container's Shape
Fills Space
When matter absorbs energy, its atoms move faster and they spread apart from each other. Atoms are packed closest together in solids and farthest apart in gases.
Solid

Liquid

Metals, Nonmetals, and Metalloids

Metals are shiny and can be bent or pulled. They are good conductors of heat and electricity. Many of them are solids at room temperature.
Nonmetals are dull, brittle, and por conductors of heat and electricity. Many of them are gases at room temperature
Metalloids are conductors of electricity under some conditions
Tuesday, March 11, 2008
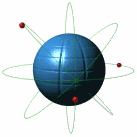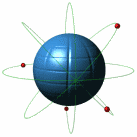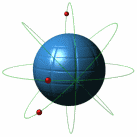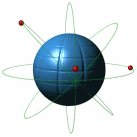
Atoms
Atoms are the very small substances of all matter. The nucleus is the central part of the atom and contains most of its mass. An atom has three main parts; the proton(+), neutron(no charge), and electron(-)
Matter
This blog is about matter,atoms,the periodic table etc. Our project will show how to arrange rows and column in the periodic table. Also about chemical reactions and physical change.
Subscribe to:
Posts (Atom)